2026 Author: Howard Calhoun | [email protected]. Last modified: 2025-01-24 13:10:31
Hydrogen is a clean fuel as it produces only water and provides clean energy using renewable energy sources. It can be stored in a fuel cell that produces electricity using an electrochemical conversion device. Hydrogen is the source of the revolutionary energy of the future, but its development is still very limited. Reasons: energy that is difficult to produce, cost effectiveness and questionable energy balance due to the energy intensive nature of the design. But this energy option offers interesting perspectives in terms of energy storage, especially when it comes to renewable sources.
Fuel Cell Pioneers

The concept was effectively demonstrated by Humphry Davy in the early nineteenth century. This was followed by the pioneering work of Christian Friedrich Schonbein in 1838. In the early 1960s, NASA, in collaboration with industrial partners, began developing generatorsof this type for manned space flights. This resulted in the first block of PEMFC.
Another GE researcher, Leonard Nidrach, has upgraded Grubb's PEMFC using platinum as a catalyst. Grubb-Niedrach was further developed in collaboration with NASA and was used by the Gemini space program in the late 1960s. International Fuel Cells (IFC, later UTC Power) developed the 1.5 kW device for Apollo space flights. They provided electricity as well as drinking water for the astronauts during their mission. IFC subsequently developed the 12kW units used to provide onboard power for all spacecraft flights.
The automotive element was first invented by Grulle in the 1960s. GM used Union Carbide in an "Electrovan" car. It was only used as a company car, but could travel up to 120 miles on a full tank and reach speeds of up to 70 miles per hour. Kordesch and Grulke experimented with a hydrogen motorcycle in 1966. It was a cell hybrid with a NiCad battery in tandem that achieved an impressive 1.18L/100km. This move has advanced e-bike technology and the commercialization of e-motorcycles.
In 2007, fuel sources became commercialized in a wide variety of areas, they began to be sold to end users with written warranties and service capabilities, i.e. meet the requirements and standards of a market economy. Thus, a number of market segments began to focus on demand. In particular, thousands of auxiliary powerPEMFC and DMFC (APU) units have been commercialized in entertainment applications: boats, toys and training kits.
Horizon in October 2009 showed the first commercial Dynario electronic system that runs on methanol cartridges. Horizon fuel cells can charge mobile phones, GPS systems, cameras or digital music players.
Hydrogen production processes

Hydrogen fuel cells are substances that contain hydrogen as fuel. Hydrogen fuel is a zero-emission fuel that releases energy during combustion or through electrochemical reactions. Fuel cells and batteries produce electricity through a chemical reaction, but the former will produce power as long as there is fuel, thus never losing charge.
Thermal processes to produce hydrogen typically involve steam reforming, a high-temperature process where steam reacts with a hydrocarbon source to release hydrogen. Many natural fuels can be reformed to produce hydrogen.
Today approximately 95% of hydrogen is produced from gas reforming. Water is split into oxygen and hydrogen by electrolysis, in a device that functions like a Horizon zero fuel cell in reverse.
Solar based processes

They use light as an agent to produce hydrogen. Existsseveral processes based on solar panels:
- photobiological;
- photoelectrochemical;
- sunny;
- thermochemical.
Photobiological processes use the natural photosynthetic activity of bacteria and green algae.
Photoelectrochemical processes are specialized semiconductors for separating water into hydrogen and oxygen.
Thermochemical hydrogen solar production uses concentrated solar energy for water separation reaction along with other species such as metal oxides.
Biological processes use microbes such as bacteria and microalgae and can produce hydrogen through biological reactions. In microbial biomass conversion, microbes break down organic matter such as biomass, while in photobiological processes, microbes use sunlight as a source.
Generation components

Devices of elements are made of several parts. Each has three main components:
- anode;
- cathode;
- conductive electrolyte.
In the case of Horizon fuel cells, where each electrode is made of a high surface area material impregnated with a platinum alloy catalyst, the electrolyte material is a membrane and serves as an ion conductor. Electrical generation is driven by two primary chemical reactions. For elements using pureH2.
Hydrogen gas at the anode splits into protons and electrons. The former are carried through the electrolyte membrane, and the latter flow around it, generating an electric current. Charged ions (H + and e -) combine with O2 at the cathode, releasing water and heat. The many environmental issues that affect the world today are mobilizing society to achieve sustainable development and progress towards protecting the planet. Here in the context, the key factor is the replacement of the actual basic energy resources with others that can fully satisfy human needs.
The elements in question are just such a device, thanks to which this aspect finds the most likely solution, since it is possible to obtain electrical energy from clean fuel with high efficiency and without CO emissions2.
Platinum catalysts

Platinum is highly active for hydrogen oxidation and continues to be the most common electrocatalyst material. One of Horizon's main areas of research using platinum-reduced fuel cells is in the automotive industry, where engineered catalysts made from platinum nanoparticles supported on conductive carbon are planned in the near future. These materials have the advantage of highly dispersed nanoparticles, high electrocatalytic surface area (ESA), and minimal particle growth at elevated temperatures, even at higher Pt loading levels.
Pt-containing alloys are useful for devices running on specialized fuel sources such as methanol or reforming (H2, CO2, CO and N2). The Pt/Ru alloys have shown improved performance over pure electrochemical Pt catalysts in terms of methanol oxidation and no possibility of carbon monoxide poisoning. Pt 3 Co is another catalyst of interest (especially for Horizon fuel cell cathodes) and has shown improved oxygen reduction reaction efficiency as well as high stability.
Pt/C and Pt 3 Co/C catalysts showing highly dispersed nanoparticles on surface carbon substrates. There are several key requirements to consider when choosing a fuel cell electrolyte:
- High proton conductivity.
- High chemical and thermal stability.
- Low gas permeability.
Hydrogen energy source
Hydrogen is the simplest and most abundant element in the universe. It is an important component of water, oil, natural gas and the whole living world. Despite its simplicity and abundance, hydrogen is rarely found in its natural gaseous state on Earth. It is almost always combined with other elements. And it can be derived from oil, natural gas, biomass, or by separating water using solar or electric energy.
Once hydrogen is formed as molecular H2, the energy present in the molecule can be released by interactionwith O2. This can be achieved with either internal combustion engines or hydrogen fuel cells. In them, the energy H2 is converted into electric current with low power losses. Thus, hydrogen is an energy carrier for moving, storing and delivering energy produced from other sources.
Filters for power modules

Obtaining alternative energy elements is impossible without the use of special filters. Classic filters help in the development of power modules of elements in different countries of the world due to high quality blocks. Filters are supplied to prepare fuel such as methanol for cell applications.
Typically applications for these power modules include power supply in remote locations, backup power for critical supplies, APUs on small vehicles and marine applications such as Project Pa-X-ell which is a project to test cells on passenger ships.
Stainless steel filter housings that solve filtration problems. In these demanding applications, zero dawn fuel cell manufacturers are specifying Classic Filters stainless steel filter housings due to production flexibility, higher quality standards, fast deliveries and competitive prices.
Hydrogen technology platform
Horizon Fuel Cell Technologies was founded in Singapore in 2003 and today has 5 international subsidiaries. The mission of the firm isto make a difference in fuel cells by working globally to achieve rapid commercialization, lower technology costs and eliminate age-old barriers to hydrogen supply. The firm started with small and simple products that require low amounts of hydrogen in preparation for larger and more complex applications. By following strict guidelines and a roadmap, Horizon has quickly become the world's largest sub-1000W bulk cell manufacturer, serving customers in over 65 countries with the widest selection of commercial products in the industry.
The Horizon technology platform consists of: PEM - Horizon zero dawn fuel cells (microfuel and stacks) and their materials, hydrogen supply (electrolysis, reforming and hydrolysis), hydrogen storage devices and devices.
Horizon has released the world's first portable and personal hydrogen generator. The HydroFill station can generate hydrogen by decomposing water in a tank and storing it in HydroStick cartridges. They contain an absorbent alloy of hydrogen gas to provide solid storage. The cartridges can then be inserted into a MiniPak charger that can handle small fuel filter elements.
Horizon or home hydrogen
Horizon Technologies launches hydrogen charging and energy storage system for home use, saving energy at home to charge portable devices. Horizon distinguished itself in 2006 with a toy "H-racer", a small hydrogen-powered car voted "best invention" of the year. Horizon offersdecentralize energy storage at home with its Hydrofill hydrogen charging station, which is able to recharge small portable and reusable batteries. This hydrogen plant requires only water to run and generate power.
Work can be provided by the grid, solar panels or a wind turbine. From there, hydrogen is extracted from the station's water tank and stored in solid form in small metal alloy cells. The Hydrofill Station, retailing for around $500, is an avant-garde solution for phones. Where to find Hydrofill fuel cells at this price is not difficult for users, you just need to ask the appropriate request on the Internet.
Car hydrogen charging

Like battery-powered electric cars, those powered by hydrogen also use electricity to drive the car. But instead of storing this electricity in batteries that take hours to charge, the cells generate energy on board the car by reacting hydrogen and oxygen. The reaction takes place in the presence of an electrolyte - a non-metallic conductor, in which the electrical flow is carried by the movement of ions in devices where Horizon zero fuel cells are equipped with proton-exchange membranes. They function as follows:
- Hydrogen gas is supplied to the "-" anode (A) of the cell, and oxygen is directed to the positive pole.
- On the anode the catalyst is platinum,discards electrons from hydrogen atoms, leaving "+" ions and free electrons. Only ions pass through the membrane located between the anode and cathode.
- Electrons create electric current by moving along an external circuit. At the cathode, electrons and hydrogen ions combine with oxygen to produce water flowing out of the cell.
Until now, two things have hindered large-scale production of hydrogen-powered vehicles: cost and hydrogen production. Until recently, the platinum catalyst, which splits hydrogen into an ion and an electron, was prohibitively expensive.
A few years ago, hydrogen fuel cells cost about $1,000 for every kilowatt of power, or about $100,000 for a car. Various studies were carried out to reduce the cost of the project, including replacing the platinum catalyst with a platinum-nickel alloy that is 90 times more efficient. Last year, the US Department of Energy reported that the cost of the system had dropped to $61 per kilowatt, still uncompetitive in the automotive industry.
X-ray computed tomography
This non-destructive testing method is used to study the structure of a two-layer element. Other methods commonly used to study structure:
- mercury intrusion porosimetry;
- atomic force microscopy;
- optical profilometry.
The results show that the porosity distribution has a solid basis for calculating thermal and electrical conductivity, permeability anddiffusion. Measuring the porosity of elements is very difficult due to their thin, compressible and inhomogeneous geometry. The result shows that the porosity decreases with GDL compression.
The porous structure has a significant impact on the mass transfer in the electrode. The experiment was carried out at various hot pressing pressures, which ranged from 0.5 to 10 MPa. The performance is mainly dependent on the platinum metal, the cost of which is very high. Diffusion can be increased through the use of chemical binders. In addition, temperature changes affect the lifetime and average performance of the element. The degradation rate of high temperature PEMFCs is initially low and then rapidly increases. This is used to determine the formation of water.
Problems of commercialization
To be cost-competitive, fuel cell costs must be halved and battery life similarly extended. Today, however, operating costs are still much higher, as hydrogen production costs are between $2.5 and $3, and hydrogen supplied is unlikely to cost less than $4/kg. In order for the cell to compete effectively with batteries, it should have a short charge time and minimize the battery replacement process.
Currently, polymer fuel cell technology will cost US$49/kW when mass-produced (at least 500,000 units per year). However, in order to compete with carsinternal combustion, automotive fuel cells should reach about $36/kWh. Savings can be achieved by reducing material costs (in particular, the use of platinum), increasing power density, reducing system complexity and increasing durability. There are several challenges to commercializing the technology on a large scale, including overcoming a number of technical barriers.
Technical challenges of the future
The cost of a stack depends on the material, technique and manufacturing techniques. The choice of material depends not only on the suitability of the material for the function, but also on the workability. Key tasks of the elements:
- Reduce electrocatalyst load and increase activity.
- Improve durability and reduce degradation.
- Optimization of electrode design.
- Improve the tolerance of impurities at the anode.
- Selection of materials for components. It is based primarily on cost without sacrificing performance.
- System fault tolerance.
- The performance of the element depends mainly on the strength of the membrane.
The main GDL parameters that affect cell performance are reagent permeability, electrical conductivity, thermal conductivity, and mechanical support. GDL thickness is an important factor. A thicker membrane provides better protection, mechanical strength, longer diffusion paths, and greater thermal and electrical resistance levels.
Progressive trends

Among the various types of elements, PEMFC is adapting more mobile applications (cars, laptops, mobile phones, etc.), therefore, is of increasing interest to a wide range of manufacturers. In fact, PEMFC has many advantages such as low operating temperature, high current density stability, light weight, compactness, low cost and volume potential, long service life, fast startups, and suitability for intermittent operation.
PEMFC technology is well suited to a variety of sizes and is also used with a variety of fuels when properly processed to produce hydrogen. As such, it finds use from the small subwatt scale all the way up to the megawatt scale. 88% of total shipments in 2016-2018 were PEMFC.
Recommended:
Thermal imaging control of electrical equipment: concept, principle of operation, types and classification of thermal imagers, features of application and verification
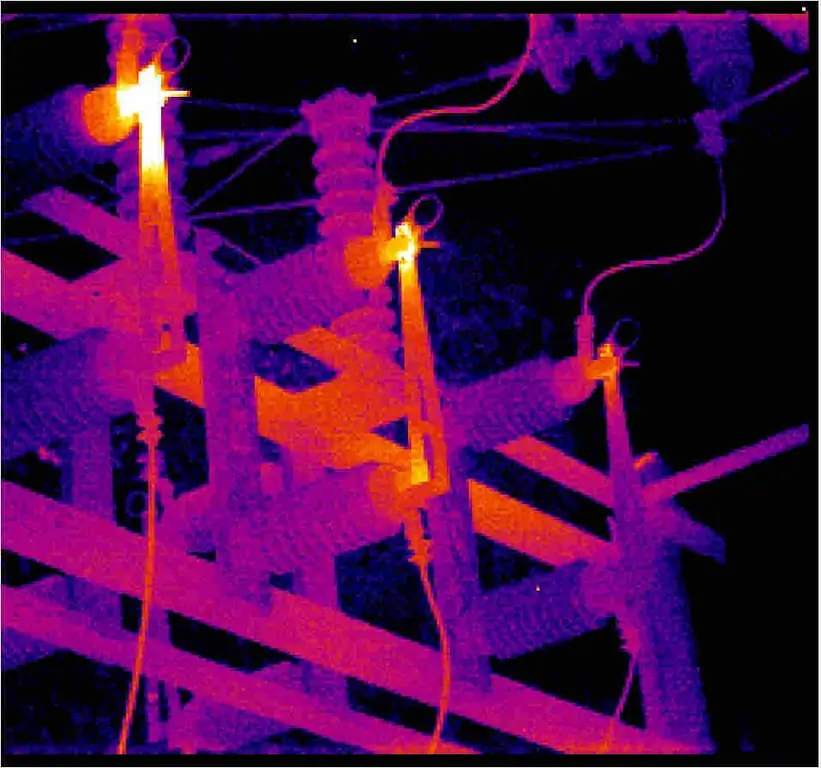
Thermal imaging control of electrical equipment is an effective way to identify defects in power equipment that are detected without shutting down the electrical installation. In places of poor contact, the temperature rises, which is the basis of the methodology
Electric locomotive 2ES6: history of creation, description with photo, main characteristics, principle of operation, features of operation and repair

Today, communication between different cities, passenger transportation, delivery of goods is carried out in a variety of ways. One of these ways was the railroad. Electric locomotive 2ES6 is one of the types of transport that is currently actively used
Low pressure heaters: definition, principle of operation, technical characteristics, classification, design, operation features, application in industry

Low pressure heaters (LPH) are currently used quite actively. There are two main types that are produced by different assembly plants. Naturally, they also differ in their performance characteristics
Scraper conveyor: principle of operation, types, purpose and features

Scraper conveyors have become widespread in the coal industry. They can move the load along a fixed chute with the help of scrapers, which are connected by a movable chain. These conveyors are used to transport dusty, granular and lumpy goods
Motor overload protection: principle of operation, features and types

Protecting an electric motor from overload is one of the most important tasks that must be solved before starting its operation. This is also important because it will help to avoid downtime during repairs. Today, there are many ways to protect

