2026 Author: Howard Calhoun | calhoun@techconfronts.com. Last modified: 2025-01-24 13:10:33
For iron smelting in the modern metallurgical industry, a blast furnace is used. This is a shaft-type furnace, which is not a very complex structure, which, however, looks impressive. To bring the production of iron to perfection, mankind had to accumulate centuries of experience.
Partly explains what a blast furnace is, the Old Slavonic root of its name. "Dmit" means to blow.
Ancestors of blast furnaces - shukofen

In the Middle Ages, people needed different metals. Weapons and tools were made of steel, flexible and hard, and ordinary iron was used for household utensils. Cheese-blast furnaces have been used to obtain the desired metal for a very long time, for millennia, and they fully satisfied the needs until the reserves of low-melting ores were depleted. A high temperature was achieved by increasing the height (this is how the thrust increased), the air was also pumped with hand bellows. However, over time, Europeans had to switch to lower quality raw materials, which served as an incentive for progress. Shtukofen became the invention after which the first blast furnace appeared. It was a closed type oven, which worked according tocertain cycle. It was necessary to load ore, coal into it, then heating with blowing took place (there was not enough manual effort, so a drive from water wheels was used), after which it was necessary to wait for cooling and extract the metal, separating it from scale and other unsuitable by-products called kritz. The main advantage of shtukofen was the best concentration of thermal energy due to the closed volume during the working cycle, due to a decrease in its leakage into the atmosphere.
Cast Iron Civilization
The main problem of medieval metallurgists in the thirteenth century was the infusibility of iron. When cast iron (that is, an iron-carbon alloy with a carbon concentration of 1.7% and higher) was obtained in shtukofen, they were surprised at its low melting point, but they were not delighted. The resulting metal was easier to obtain than steel, and even more so iron, but its mechanical qualities from the point of view of the then consumers left much to be desired: it was too fragile and not strong enough. However, in just two centuries, attitudes towards cast iron have changed. Firstly, it turned out to be a simple matter to remove it from the furnace, it could simply be drained in molten form. Secondly, this metal has nevertheless found its application, and it is very diverse. And thirdly, it was a raw material for further purification from excess carbon, and it turned out to be much easier to obtain steel from it than from ore. So, after centuries of experiments, the most productive metallurgical technology was found, and a blast furnace was invented. Oven in the Westphalian city of Siegerland (second half of the 15thcentury) could work with a continuous cycle for several years, producing more than one and a half tons of pig iron daily. It was a lot back then.
Building a blast furnace
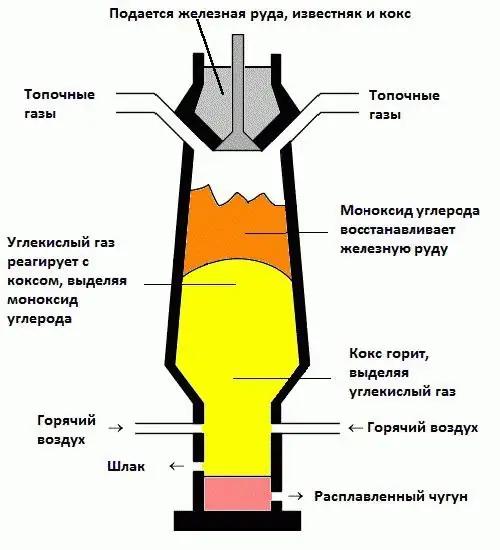
Only by being close to this giant furnace, you can understand how big the modern blast furnace is. Photographs give an idea of her cyclopean size only when they show a person who seems as small as an ant. However, despite the impressive appearance, the principle of operation remained the same, medieval. The design includes five main nodes. The upper one, the top, is designed to load raw materials and distribute them evenly inside the furnace. Below it is a part of the conical shape in which heating and the reduction process take place (more on that later). The third unit from the top is called steam, where iron is melted. Then another conical part, this time tapering downwards, is the shoulders, in which carbon monoxide (reducing gas) is released from the coke. And at the very bottom is a forge from which the finished product and production waste are extracted.

Process chemistry
Chemical processes are oxidative and reductive. The first means connection with oxygen, the second, on the contrary, its rejection. Ore is an oxide, and in order to obtain iron, a certain reagent is required that can "select" extra atoms. The most important role in this process is played by coke, which during combustion releases a large amount of heat and carbon dioxide, which at high temperature decomposes into monoxide, chemicallyactive and unstable substance. CO tends to become dioxide again, and, meeting with ore molecules (Fe2O3), “takes away” all oxygen from them, leaving only iron. There are, of course, other substances in the raw materials, unnecessary, which form a waste product called slag. This is how a blast furnace works. This is, from the point of view of chemistry, a fairly simple reduction reaction, accompanied by the consumption of heat.

What is a modern blast furnace like?
The service life of a blast furnace is relatively short for a facility of this magnitude - about a decade. During this time, the structure is subjected to heavy loads, aggravated by thermal heating, then major repairs or demolition are required. Iron production cannot be called harmless, it is associated with emissions of phosphorus, sulfur and other not very useful substances into the atmosphere. Taken together, these circumstances encourage many countries to curtail metallurgical production or modernize it (especially if the industry is basic and budget-forming). A modern blast furnace is a fairly simple system in principle, which, however, requires a complex control scheme with numerous control loops that ensure the most efficient consumption of raw materials and energy resources.
Recommended:
Glass furnace: types, device, specifications and practical application

Today, people actively use glass for a variety of purposes. The glassmaking process itself is the melting of raw materials or charge. Glass melting furnaces are used to melt the material. They come in different types and are classified according to several criteria
Arc steel furnace: device, operating principle, power, control system

Arc steel-smelting furnaces (EAFs) differ from induction furnaces in that the loaded material is directly subjected to electrical bending, and the current at the terminals passes through the charged material
Steel smelting: technology, methods, raw materials

Iron ore is obtained in the usual way: open or underground mining and subsequent transportation for initial preparation, where the material is crushed, washed and processed
Granulated blast-furnace slag: production, composition, GOST

The powerful development of metallurgy has led to the fact that a wide variety of raw materials have been used in this area. Naturally, after all operations, waste remains. Blast furnace slag also belongs to this category. Despite this, it is still usable
Open-hearth furnace and its importance in steel production

The open-hearth furnace, invented in the middle of the century before last, made a real revolution and a technological breakthrough in the field of ferrous metallurgy. There was an opportunity for the production of steel on an industrial scale. This was the starting point for the rapid development of mechanical engineering. Many objects and mechanisms that we regularly use, without thinking about the history of their creation, began their journey in the hearth of an open-hearth furnace

